April 14, 2022
Leader in the technological innovation of spinal surgery
Medical Devices
Life Science

Tsunami Medical s.r.l with its latest generation implantable products was the protagonist of the inaugural event of the Medical Devices Virtual Talks of JSB Solutions.
Founded in 1997 in the heart of the Biomedical Valley, near Modena, the company is now a leader in the technological innovation of spinal surgery, with a main focus on medical solutions made using the technology of 3D printing titanium. A professional reality that has 20 years of experience in the design and production of high quality medical devices, with an international calibre distribution.
With the new Regulation on Medical Devices (EU 2017/745), all products of Tsunami Medical will pass from class IIB to class III with the exception of some, such as screws, bars and devices with battery and engine, which will remain in class IIB.
The products meet current trends in international markets by offering high-quality spinal surgical solutions: medical devices that comply with and meet the regulatory requirements and international quality standards imposed, not only to make a difference in the biomedical sector, but above all in people’s lives. The company aims every day to study and propose different and increasingly innovative offers to customers in order to improve their mobility and quality of life.
Initially the company was mainly involved in the production of invasive diagnostic devices as a subcontractor of some large manufacturing companies; over the years it grew until the acquisition of the Bloodline brand, well known in the international markets of biopsy and spinal-plastic vertebrae.
Since 2010 Tsunami Medical has started the design and production of the Selective Laser Melting (SLM) technology, which then led to the realization of the vast portfolio of implantable products currently available.
The classification of a medical device must necessarily take place upstream of the project because according to the class, all the requirements, guidelines and regulations to be respected will be defined.
The classes of medical devices are defined and framed on the basis of 22 specific rules, including their purpose, their risk of use, the duration of exposure, invasiveness and energy:
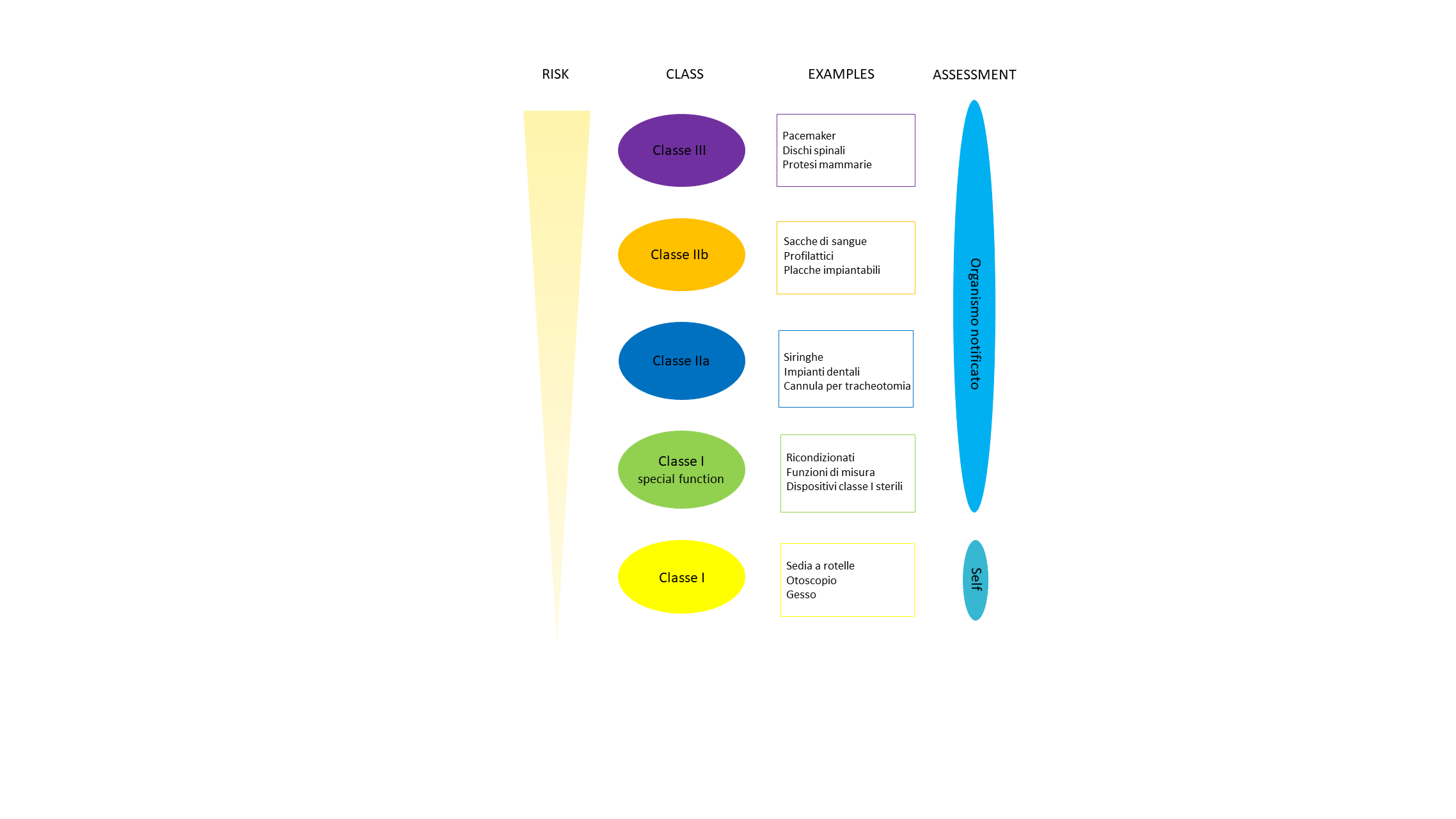
*The information shown in the graph relates to generic cases. It is recommended to carefully evaluate the specific characteristics of your device before classification.
Scopri di più
Vai al case study