April 16, 2025
Regulatory Affairs
Quality Assurance
Clinical Supply Chain
Events

For years, pharmaceutical serialization has been on the horizon of our industry - a familiar concept, discussed at conferences and anticipated in regulations. Yet, only now that we see it taking concrete form in our processes are we fully grasping its transformative potential. This isn't simply about placing a code on a package, but about building a digital ecosystem that protects patient health and generates tangible value throughout the entire supply chain.
In the journey from factory to pharmacy, each medication will tell its authenticity story through a unique code - a digital diary that allows tracking the medicine through every phase of its journey and verifying its identity at the point of dispensation. This means not only guaranteeing product authenticity, but also optimizing processes, preventing distribution shortages, and creating new opportunities for operational efficiency.
This expanded awareness clearly emerged from the pharmaceutical serialization webinar organized by JSB Solutions in collaboration with Pharma Partners: the evolution from a simple regulatory obligation to a powerful value generator for the entire supply chain. A revolution that goes well beyond combating counterfeiting, opening up previously unexplored scenarios for improving public health and economic sustainability.
On April 22, our team brought together three leading experts to decode the complexity of pharmaceutical serialization through complementary perspectives: technical-operational, regulatory, and practical.
Maurizio Battistini, Qualified Person and Supply Chain Expert, traced the evolution from paper stickers to data matrix, illustrating how this paradigm shift is redesigning the entire operational flow of pharmaceutical distribution in Europe.
Vittoria Barone, Regulatory Affairs Manager - Italy at JSB Solutions, guided participants through the complex regulatory path, from the implementation of the European directive to the recent Decree Law n.10/2025, translating regulatory language into concrete steps for compliance.
Chiara Mazzamuto, Quality Manager at Pharma Partners, completed the picture by sharing the field experience of those who have already implemented serialization, offering a pragmatic view of daily challenges and adopted solutions.
"Serialization arises from a fundamental problem: pharmaceutical counterfeiting and the consequent risk to patients," emphasized Battistini in his opening remarks. With the data presented, he illustrated the increase in pharmaceutical crimes, which rose from 4,405 episodes in 2018 to 6,615 in 2022, with an estimated incidence between 2-4% of global pharmaceutical trade.
Battistini explained that falsification can lead to the spread of "perfect counterfeit products, harmless counterfeits without active ingredients, criminal counterfeits, manipulated products, and imperfect counterfeits," with significant health risks. "Even a 'harmless' drug that doesn't contain the active ingredient can cause enormous damage to those who need it," he specified.
The European response was the creation of an end-to-end system that tracks the medicine from production to dispensation, through a unique identifier and an anti-tampering device, supported by a centralized digital infrastructure.
Battistini offered an illuminating comparison between the current Italian system and the European one. "The Italian system is based on the sticker (bollino), an identification tool that identifies products by batch and not by individual unit," he explained, describing the current approach as "a hybrid system between track and trace and end-to-end, limited to the batch number."
He illustrated how the sticker, produced by the State Printing Office and Mint, primarily serves to analyze pharmaceutical spending and monitor distribution shortages, financed by the cost of the stickers for the producer (about €0.024 per sticker).
In contrast, in the European system, "the product is tracked from the moment the code is applied until it's dispensed to the patient, with an authenticity verification before dispensation," Battistini clarified. The identification code is a data matrix printed directly on the package, not an applied label, and data transmission occurs in real-time.
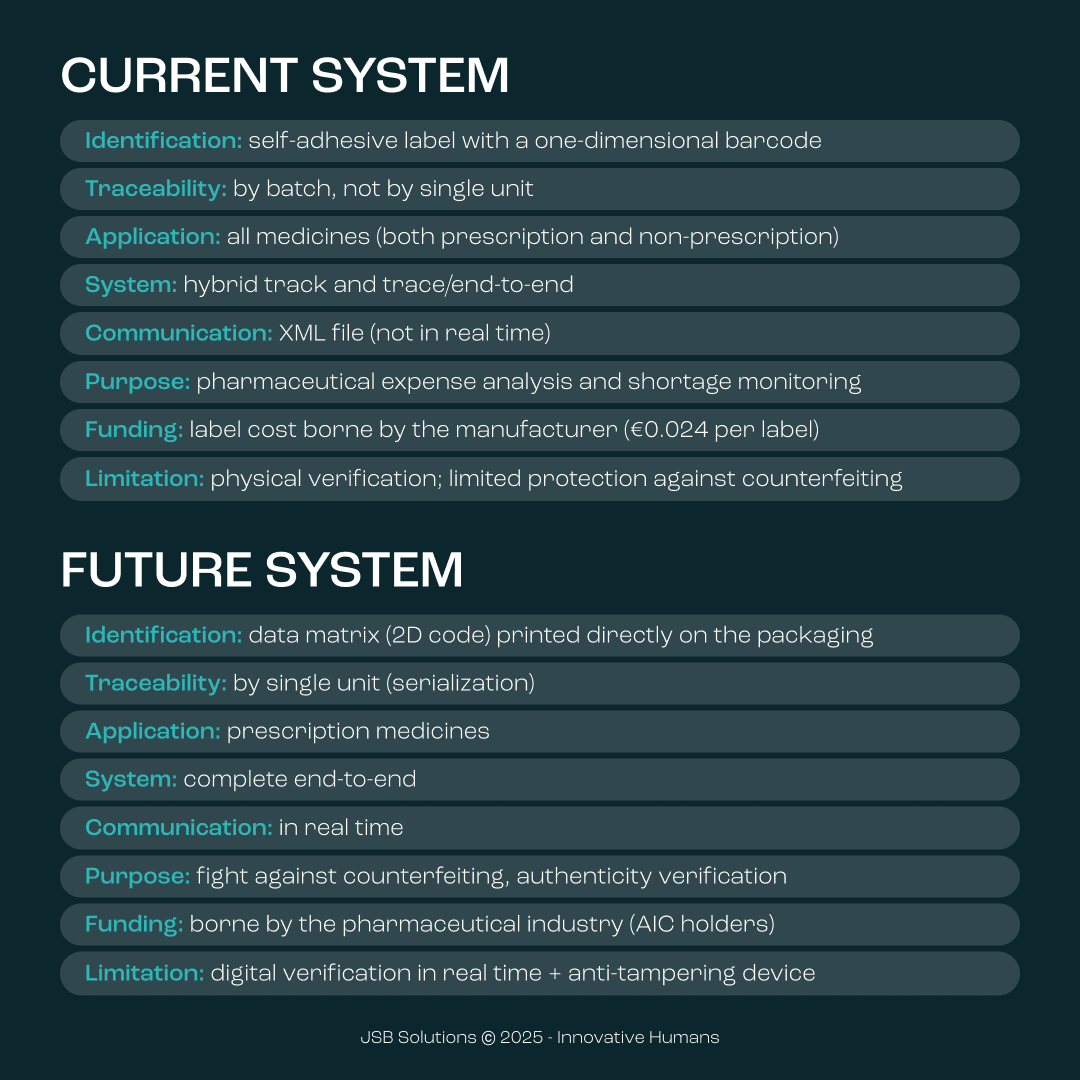
Vittoria Barone illustrated how Italy represents a special case in the European landscape: "While most European Union countries have implemented serialization since 2019, Italy - thanks to its pioneering sticker systems already in operation - obtained, along with Greece, a six-year waiver to progressively adapt to the new European system, with the 'go-live' set for February 9, 2025."
She illustrated how the recent Decree Law n.10/2025 marks the beginning of Italy's journey toward European alignment, providing for a "24-month stabilization period" until February 8, 2027. "The decree introduces not only the European data matrix and anti-tampering device but also a distinctive Italian element: a device made on security support, the so-called 'value paper', produced by the State Printing Office and Mint," she clarified.
Barone added that during this transitional period, "prescription medications can be released with the current sticker or, when the NMVS becomes operational, with data matrix, anti-tampering device, and security support device."

Chiara Mazzamuto shared Pharma Partners' direct experience in implementing serialization since 2019. "Serialization can be implemented in two distinct ways: on the flat package, upstream of the production process, or downstream of the production process, on the already packaged box," she explained.
She illustrated the fundamental steps: defining serialization specifications, developing connections for data transfer between companies, and executing the process, delving into the specifics of each phase.
Regarding issues in data matrix management, Mazzamuto indicated the most common difficulties: "incorrect order of application identifiers, use of the wrong application identifier, incorrect use of separators, and non-compliant grade (print quality level)." She specified that the grade has a scale from four to zero, and "at the European regulatory level, a reading tolerance down to 1.5 is allowed; below this value, it is considered not sufficiently readable."
On serial management during the production process, she highlighted attention points such as "manual or machine rejection that ends up among the good ones" and the importance of "100% presence of tamper evident on all packages," describing various control strategies.
"The strategic integration of serialization data with digital technologies transforms an obligation into a concrete competitive advantage," we emphasized in a recent LinkedIn post. This concept was explored during the webinar, highlighting the benefits of serialization:
- Counterfeiting prevention
- Patient protection
- Strengthening supply chain control
- Improving operational efficiency
- Automating regulatory reporting
As Battistini observed, "some producers, especially those already producing for other European markets, are more aware of these aspects compared to local producers." The data generated by serialization represents a strategic resource still largely unexplored.
The conclusion of the webinar led to a collective reflection on future challenges, where technical, regulatory, and operational perspectives intertwined in a complete and multifaceted picture.

"European experiences have already revealed some critical issues that we must carefully consider," emphasized Battistini. "Misalignments between databases and management of false positives have been concrete challenges in various countries." The comparison with realities such as Germany, Spain, and France demonstrates the importance of planning every aspect of interoperability, from system synchronization to personnel training. "The real challenge will not only be technological," he added, "but will require a rethinking of the entire approach to traceability, potentially integrating emerging technologies such as RFID, blockchain, and artificial intelligence to facilitate data migration and authentication."
On the regulatory front, the establishment of the EMVO represents a fundamental organizational infrastructure to manage the Italian transition. "The technical table established in March represents a crucial element of this process," highlighted Barone. "It will operate throughout the stabilization period, drafting semi-annual reports on progress, allowing us to monitor and intervene promptly." The twelve implementing decrees envisaged will not be mere bureaucratic fulfillments but tools to systematically address criticalities. "It's not just about adapting to a regulation," she specified, "but about progressively calibrating the integration between the pre-existing Italian system and the new European requirements."
From a practical implementation perspective, pharmaceutical companies must prepare for significant changes in production. "Artwork revision will require considerable time and resources," noted Mazzamuto. "I wouldn't underestimate the introduction of the value paper, which will involve new assessments on material machinability and possible adjustments in production processes." The challenge will not only be technical but also organizational. "It will be necessary to develop new internal skills," she concluded, "and establish optimized connections for data transfer among all supply chain actors. Those who start planning today will have a competitive advantage tomorrow."
This convergence of technical, regulatory, and operational challenges will require an integrated approach and close collaboration among all sector stakeholders, from pharmaceutical companies to regulatory authorities, from technology providers to specialized consultants. As Battistini noted in his concluding remarks: "Those who can transform this obligation into a strategic opportunity will reap benefits well beyond mere regulatory compliance. The data generated by serialization is a gold mine still largely unexplored."
The webinar concluded with a stimulating question and answer session that allowed delving into specific aspects of serialization implementation.
A participant raised a question about managing medicines destined for export to non-EU countries, an increasingly relevant topic in an increasingly globalized pharmaceutical market.
Battistini explained the fundamental principle: "A product introduced into the system must be removed at the moment it exits the control chain of the system itself." However, he admitted that "data transfer for extra-EU exports has often proven complicated" and that "it has often been necessary to perform decommissioning in the country of origin and then reload the product with the destination country codes," highlighting the lack of "complete alignment of rules among various non-EU countries."
To complement this response, Vittoria Barone added a crucial point: "A drug cannot simply be sent outside the community without being decommissioned at the community level, otherwise it would remain registered in the European system without traceability in the extra-EU destination."
Another question concerned the management of free samples in the new regulatory context, a topic of particular interest to pharmaceutical companies that use this practice.
Vittoria Barone clarified that "the flow for free samples should be similar to the current one." She then specified that "for samples classified as SOP and OTC, the current sticker and anti-tampering device as per European regulation will be used," while "for prescription free samples (class H and C), the complete serialization system will apply with data matrix, value paper, and anti-tampering device."
A participant raised a practical question regarding payment of the entrance fee for the ENVW, a non-secondary economic aspect of implementation.
Vittoria Barone confirmed that "marketing authorization holders have received a proforma invoice via certified email for the entrance fee, with a 30-day deadline from receipt." She specified that "while not a proper invoice, it is necessary to respect this deadline as this fee serves to support the start-up costs of the ENVW platform."
A technical question led to clarification on the expiry date format in the data matrix, a critical element for correct implementation.
Chiara Mazzamuto confirmed that "in countries where Pharma Partners already performs serialization, within the data matrix the expiry date is expressed with six digits (year year, month month, day day)." She specified that "usually for the day, the last day of the month is indicated, following the client's instructions," an important detail for uniformity of information in the system.
A final question concerned the parallel with Greece, the other European country that benefited from a waiver for serialization implementation.
Battistini explained that "Greece, like Italy, had obtained a waiver until February 9, 2025, thanks to its pre-existing traceability systems." He specified that "while Italy has formalized a two-year transition phase until 2027, Greece has not yet provided formal information on a similar extension."
He then detailed the steps taken by Greece, which "established the Hellenic Medicines Verification Organization (HMVO) in November 2023" and "signed an agreement for the supply of the national verification system (MVS) in 2024." Additionally, "it is working to connect the HNVO and pharmacies to integrate the serialization system into the reimbursement process." However, he concluded, "despite these practical advances, Greece has not started implementation as planned and has not officially communicated its future plans."
The complexity of this transition requires much more than simple isolated technical consulting. At JSB Solutions, we have developed a holistic vision that embraces the entire serialization ecosystem, where regulatory, technical, and operational expertise constantly dialogue.
Unlike traditional fragmented approaches that address single aspects of the problem (only regulations, only technology, only production processes), our multidisciplinary team offers an integrated solution that accompanies the client throughout the entire journey.
This synergy between our various areas of expertise - from regulatory affairs to quality assurance, from IT to supply chain - allows identifying connections and opportunities that would otherwise remain invisible.
Companies collaborating with us benefit not only from impeccable regulatory compliance but from a strategic vision that transforms serialization into a long-term competitive asset. The data generated by traceability systems thus becomes not a mere fulfillment but a valuable resource for optimizing processes, improving transparency with customers, and strengthening pharmaceutical supply chain security.
For JSB Solutions, this webinar represents a further step in our commitment to guide clients through transformations in the Life Science sector, building together a safer, more efficient, and more transparent future for the entire pharmaceutical supply chain.
Scopri di più
Vai al case study